Johnson Lab
Applied Sciences | Translational Bioscience
Northumbria University
Newcastle upon Tyne, UK
Mitochondrial defects are linked to diseases ranging from neurodegenerative disease to cancer, but the precise role of mitochondria is often unclear. Defining the mitochondrial functions important for individual diseases, and the cell, tissue, nutrient, and developmental contexts linking these functions to disease, will allow us to identify new approaches for treatment.
Defining the role of mitochondria in human disease is an overarching theme of work in the Johnson laboratory.
Mitochondria in human disease
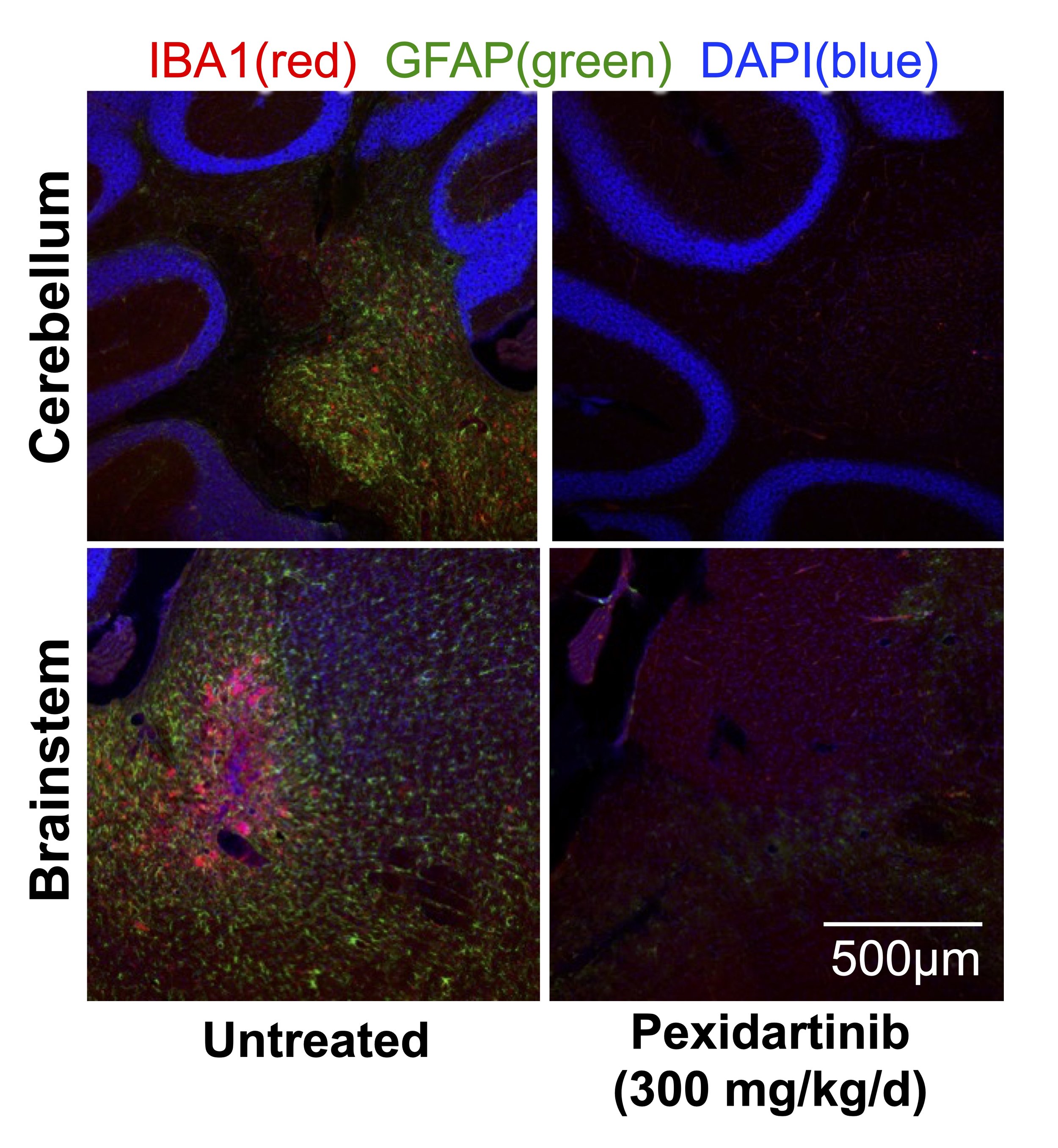
Lesions in genes coding for mitochondrial components can lead to one of many disorders collectively termed genetic mitochondrial diseases (GMDs).
GMDs are remarkably genetically and clinically heterogeneous. Over 250 unique genes have been causally linked to GMD, complicating diagnosis and treatment, and limiting the potential of gene therapy approaches.
Our lab is focused on searching for common mechanisms of pathology among distinct forms of GMD to identify treatments which will benefit many patients.
Recent work (figure left from Stokes et al, 2022) in our lab has identified immune activation as a key causal pathway in the pathogenesis of the GMD Leigh syndrome.
Pathobiology of genetic mitochondrial diseases

Biogerontology
Our laboratory has a long-standing interest in biogerontology - the study of aging and age-related diseases.
In particular, we are interested in (1) the role of mitochondrial dysfunction in aging and age-related disease, (2) mechanisms underlying age-related changes to anaesthesia responses, and (3) the mechanisms underlying the benefits of mTOR inhibition in the setting of aging.
Recent work from our laboratory has revealed that mTOR inhibition benefits mitochondrial disease through actions on the immune system. We are exploring whether the benefits of targeting mTOR in aging are similarly defined by effects on the immune system. Recent preliminary data indicate that a PI3Ky/mTOR mediated immune pathway may contribute to brain aging, similar to the role of PI3Ky/mTOR in Leigh syndrome.

Basic biology of volatile anesthesia
While volatile anaesthetics (isoflurane, sevoflurane, etc) are routinely used in human medicine, precisely how they work is still not known.
In addition, while they are safe in the general public, some rare genetic defects, including mitochondrial genes, lead to remarkable hypersensitivity and anaesthetic toxicity.
Developmental state, age, and nutritional status are also factors which impact the safety and can underlie off-target effects of anesthetics. Dr’s Morgan and Sedensky, our collaborators at UW/SCRI, were the first to find that volatile anaesthetics directly impact mitochondrial function.
Our lab is working to define the mechanisms of anaesthetic toxicity in mitochondrial disease, anaesthetic sensitivity in geriatric and neonatal patients, off-target metabolic effects of anaesthesia, and the basic biology of how organisms ‘wake up’ from anaesthesia. Left: isoflurane induces mitochondrial ‘flashing’ - transient fluctuations in mitochondrial membrane potential.